pf3 electron pair geometry|10.2: VSEPR Theory : Tagatay Looking at the PF3 Lewis structure we can see that there are three Fluorine (F) atoms attached to the central Phosphorus (P) atom and that there is one lone pair of electrons . Nathan insists it wasn't supposed to be sent to his own mom, but he won't tell Syren who it was meant for instead. Eventually Syren insists on seeing Nathan's phone, at which point she realizes that the photo was meant for Nathan's best friend's mom, Lexi Luna. . Click Here For Membership To Full-Length Episode! Previous Episodes. 2 .Mustang Gold slot is a must-have slot machine in any online casino in Canada. It’s an essential Wild West-themed slot that offers plenty of opportunities to win. However, it’s crucial for players to understand what symbols are the most beneficial and how to utilize free spins before they have a chance at cracking a jackpot.
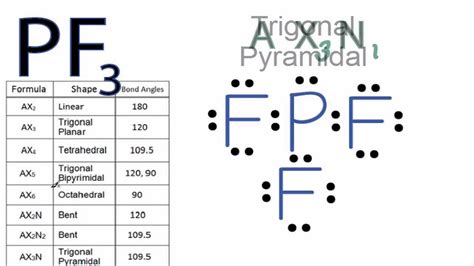
pf3 electron pair geometry,For the molecular geometry, looking at the PF3 Lewis structure we can see that there are three Fluorine (F) atoms attached to the central Phosphorus (P) atom and that there .
Determine Electron Pair Geometry. Using VSEPR (Valence Shell Electron Pair Repulsion) theory, the electron pairs around the central atom (phosphorus) will arrange . Looking at the PF3 Lewis structure we can see that there are three Fluorine (F) atoms attached to the central Phosphorus (P) atom and that there is one lone pair of electrons .The premise of the VSEPR theory is that electron pairs located in bonds and lone pairs repel each other and will therefore adopt the geometry that places electron pairs as far apart from each .
6 Steps to Draw the Lewis Structure of PF3 Step #1: Calculate the total number of valence electrons. Here, the given molecule is PF3 (phosphorus trifluoride). In order to draw .
In calculating electronic geometry we use the Valence Shell Electron Pair Repulsion (VSEPR) model, which states that the lowest geometry for electronic orbitals around a positive nucleus is for the orbitals to be as far away as possible. Valence shell electron-pair repulsion theory (VSEPR theory) enables us to predict the molecular structure, including approximate bond angles around a central atom, of a molecule or a polyatomic ion from an examination . We show you how to draw the Lewis structure and determine the moleculargeometry for phosphorus trifluoride (PF3).pf3 electron pair geometry Simple steps for drawing the Lewis dot structure for PF3. 1. Count total valence electron in PF3. In the first step, we have to find how many valence electrons are there in PF3, so that we can distribute them around central and .Phosphorus Trichloride: Phosphorus trifluoride is the name of PF 3.It's a gas that is known for its toxicity. We will use valence shell electron pair repulsion (VSEPR) theory to determine its molecular geometry. The electron geometry of PF3 is tetrahedral due to four areas of electron density according to the VSEPR theory, which include three bonding pairs and one lone pair, resulting in a trigonal pyramidal molecular geometry. Explanation: The electron geometry of PF3 is determined by the steric number, which is the sum of bonded atoms and lone pairs .
Electron-pair Geometry versus Molecular Structure. It is important to note that electron-pair geometry around a central atom is not the same thing as its molecular structure. The electron-pair geometries shown in . Very important. Clarify which type of geometry the question is asking about before answering it. The electron domain geometry is tetrahedral for both but the molecular geometry is trigonal pyramidal (not trigonal bipyramidal for PF3) for both. Both geometrical assignments are due to the lone pair of electrons on the central atom(s) of attachment. While electron geometry does include lone pairs. PCl3 has a bond angle of 103 degrees. The decrease from the ideal bond angle of trigonal pyramidal compounds (109 degrees ) is due to repulsion between the lone pairs on phosphorus. . Given below is the MO diagram of PF3 taking reference to which you can easily draw for PCl3. A MO diagram helps .
An explanation of the electron geometry for the BF3 (Boron trifluoride) . The electron geometry for the Boron trifluoride is also provided.The ideal bond ang.
Figure \(\PageIndex{5}\): (a) The electron-pair geometry for the ammonia molecule is tetrahedral with one lone pair and three single bonds. (b) The trigonal pyramidal molecular structure is determined from the electron-pair geometry. (c) The actual bond angles deviate slightly from the idealized angles because the lone pair takes up a larger .
pf3 electron pair geometry 10.2: VSEPR Theory The electron group geometry for a molecule with four electron pairs is tetrahedral, as was seen with \(\ce{CH_4}\). In the ammonia molecule, one of the electron pairs is a lone pair rather than a bonding pair. Although the lone pair is not visible, it will affects the location and bond angles among other atoms in the molecule.
PCl3 Molecular Electron Geometry, Lewis Structure, Bond Angles and Hybridization. Phosphorus trichloride is made up of one Phosphorus atom and three Chlorine atoms, having a chemical formula of PCl3. It is a volatile liquid that reacts with water and releases HCl gas. It is a toxic compound but is used in several industries.

Remember electron groups include not only bonds, but also lone pairs! Name the electron-group geometry. (State whether it is linear, trigonal-planar, tetrahedral, trigonal-bipyramidal, or octahedral.) Looking at the positions of other atomic nuclei around the central determine the molecular geometry. (See how many lone pairs there are.)
10.2: VSEPR Theory 2. Determine Electron Pair Geometry. Using VSEPR (Valence Shell Electron Pair Repulsion) theory, the electron pairs around the central atom (phosphorus) will arrange themselves to minimize repulsion. The steric number (number of bonding pairs + lone pairs) for phosphorus in PF3 is 4 (three bonding pairs + one lone pair).

Bonding electrons i.e. number of pairs. Lone pairs (non-bonding pairs: when electrons do not participate in the formation of bonding between atoms) The atomic number of boron (B) and fluorine (F) is 5 and 9 . An explanation of the molecular geometry for the SCl2 (Sulfur dichloride) including a description of the SCl2 bond angles. The electron geometry for the Sulf. The VSEPR shape of the molecule "PF"_3 is trigonal pyrimidal. We can use VESPR theory to predict a trigonal pyrimidal shape for the molecule PF_3 because of its AX_3E status. VESPR stands for valence shell electron pair repulsion. This theory basically says that bonding and non-bonding electron pairs of the central atom in a molecule will repel (push . Second, find the total electron pairs; We have a total of 26 valence electrons. And when we divide this value by two, we get the value of total electron pairs. Total electron pairs = total valence electrons ÷ 2. So the total electron pairs = 26 ÷ 2 = 13. Third, determine the central atom; We have to place the least electronegative atom at the . The electron-pair geometry and molecular structure are identical, and CO 2 molecules are linear. (b) We write the Lewis structure of BCl 3 as: Thus we see that BCl 3 contains three bonds, and there are no lone pairs of electrons on boron. The arrangement of three regions of high electron density gives a trigonal planar electron-pair geometry. Hydrogen has one valence electron in its outer shell. But there are three hydrogen atoms in this molecule, due to which we will multiply the number by 3. . PH3 Molecular Geometry and Shape. . All the bonding pairs of electrons are at the base of the pyramid of geometry, and the lone pair is at the top. Hence the molecular geometry for the .
Identify the electron-pair geometry based on the number of regions of electron density: linear, trigonal planar, tetrahedral, trigonal bipyramidal, or octahedral (Figure \(\PageIndex{2}\)). Use the number of lone pairs to assign an AX m E n designation and determine the molecular geometry. Identify the LP–LP, LP–BP, or BP–BP interactions .
pf3 electron pair geometry|10.2: VSEPR Theory
PH0 · Phosphorus Trifluoride
PH1 · PF3 lewis structure, Molecular geometry, Bond angle,
PH2 · PF3 Molecular Geometry / Shape and Bond Angles
PH3 · PF3 Lewis Structure, Molecular Geometry, and Hybridization
PH4 · PF3 Electron Geometry (Phosphorus trifluoride)
PH5 · Molecular geometry pf3
PH6 · Lewis Structure of PF3 (With 6 Simple Steps to Draw!)
PH7 · Chemistry Learning Made Easy: PF3: Lewis Structure and
PH8 · 8.6: Molecular Geometries
PH9 · 5.9: Molecular Geometry
PH10 · 10.2: VSEPR Theory